Kangen Chemistry
Most tap water in the US is hard water, which contains large amounts of dissolved mineral carbonates such as calcium carbonate. A Water ionizer uses electrically charged plates to change the mineral carbonates in ordinary tap water into mineral hydroxides. This chemical change is what makes alkaline water from a water ionizer superior to plain water.
Hard water – where alkaline water comes from
Water is known to chemists as “the universal solvent” because most substances dissolve in it. When a substance dissolves, the elements that make it become dissociated – separate from each other. For example, when you dissolve salt in water you have a solution of water, sodium ions, and chloride ions.
Example: Salt dissolved in water = NaCl + H20 Na++ + Cl— + H20
Hard water is made up mostly of calcium carbonate and magnesium carbonate. These two minerals are primarily responsible for water’s healthy qualities. Because both mineral salts are dissolved in water, hard water will contain calcium ions, magnesium ions, and carbonate ions in solution with water. Carbonates like calcium carbonate and magnesium carbonate give water close to a neutral pH.
The Chemistry of Ionized Alkaline Water
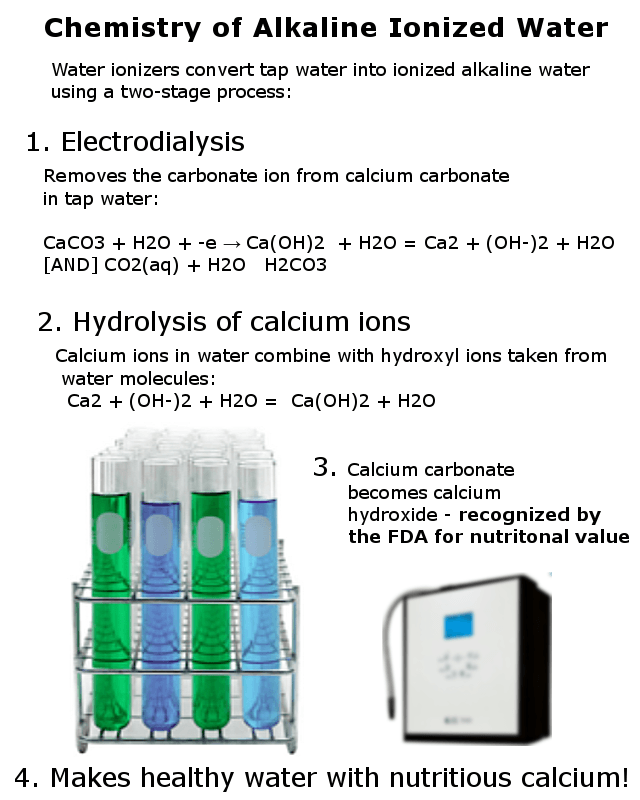
When hard water passes through a water ionizer, the calcium and magnesium ions are electrically separated from the carbonate ions. As a result, the water ionizer produces two streams of water; the alkaline stream is rich in calcium and magnesium. The acidic stream of water contains the carbonates that were split off by the water ionizer.
Aqueous calcium carbonate solution to Calcium hydroxide solution
CaCO3 + H2O + -e → Ca(OH)2 + H2O Ca++ + (OH–)2 + H2O [AND] CO2(aq) + H2O H2CO3
Hydrolysis of alkaline water minerals
Elements like calcium and magnesium aren’t stable in water. Because of this, those ions combine with a hydroxyl ion taken from the water itself, this process is called hydrolysis. The result is a known to chemists as an aqueous solution that contains mineral ions and hydroxyl ions.
Hydrolysis of calcium: Ca++ + (OH–)2 + H2O Ca(OH)2 + H2O
Hydrolysis of magnesium: Mg++ + (OH-)2 + H2O Mg(OH)2 + H2O
The mineral hydroxides in alkaline water have a higher pH than the mineral carbonates in tap water. This is why the ionized alkaline water produced by a water ionizer has a higher pH than the source water.
Calcium and Magnesium Hydroxide – Recognized by the FDA as safeCalcium and magnesium hydroxide are both recognized as safe nutritional supplements by the FDA. Calcium hydroxide is considered to be such a safe and effective nutritional supplement that it is used to fortify infant formula and orange juice with calcium!
Magnesium hydroxide is the active ingredient in Milk of Magnesia; as such it has brought relief of digestive upset to millions of people. The amount of magnesium hydroxide in alkaline water is way too small to have the same effect as Milk of Magnesia, however.
Why alkaline water from a water ionizer is better than plain waterThe mineral hydroxides in alkaline water break down in the body more readily than the mineral carbonates in plain water. That means that calcium hydroxide is more readily absorbed in the body. That is why calcium hydroxide is chosen over calcium carbonate as a calcium supplement in infant formula. The magnesium hydroxide in alkaline water is also recognized as a good source of magnesium in the diet.
You can tell the difference between alkaline water and plain water when you drink it. Alkaline water tastes sweeter and more refreshing than regular water. The mineral hydroxides in alkaline water make it taste better, and make it better for you.